
AI Enabled Content Generation for Clinical and Regulatory Documents Platform Solutions
In Part One of our three-part series, we assessed your organization’s AI readiness for automating regulatory and clinical trial document creation.
In Part Two, we explored Structured Content Authoring, defining this essential building block to establish modular frameworks representing all the data associated with clinical development.
Now, in Part Three, we examine what AI-enabled content generation truly entails, why it’s gaining momentum today, and how organizations can effectively embrace it. This AI-generated content populates the Structured Content Framework detailed in Part Two.
This series provides a comprehensive examination of how teams can streamline the composition of regulatory and clinical documents through automation, as well as how experts can guide Pharma, Biotech, and Medical Device companies on their path to substantial time and cost savings.
What is the current state of AI in clinical and regulatory content?
In 2020, Gartner® predicted that, “by 2023, life science business leaders will push AI into 50% of enterprise core processes – exposing gaps in IT architecture, governance and skills.” The reality? “The 2024 Gartner CIO and Technology Executive Survey showed that 43% of life science respondents have deployed AI/ML within their organization.” (Gartner, 2024)
Many organizations rushed into AI and machine learning without adequate preparation. They had fragmented IT architecture, gaps in processes and governance, and limited time to establish these capabilities. While interest is high, several real-world barriers continue to slow meaningful progress:
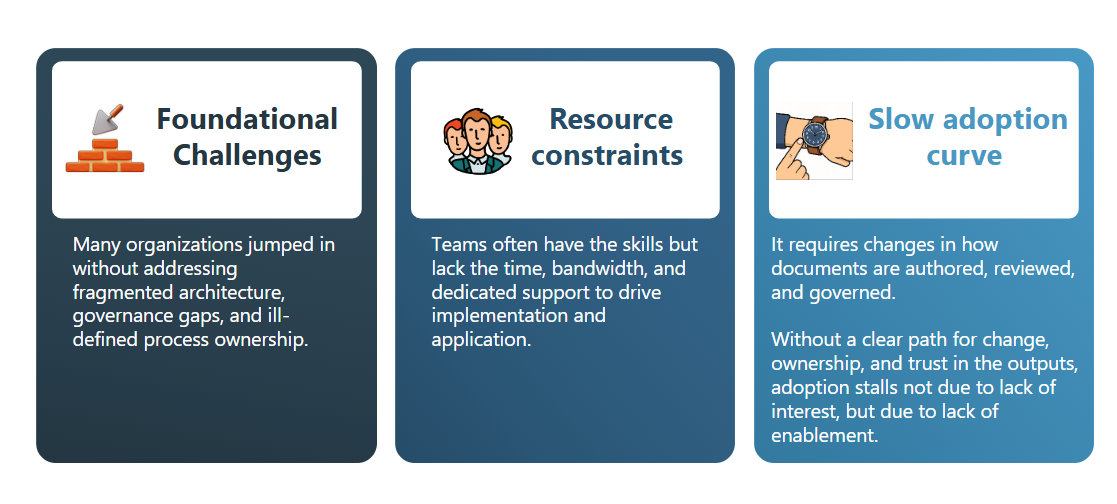
At Astrix, we help organizations shift from experimentation to adoption. Our approach aligns scalable architecture, transparent governance, and effective change management to ensure AI becomes an integral part of how work gets done, not just a side project.
What Is AI-Enabled content generation?
The prevalence of content generation tools bolsters our confidence in what they can accomplish for authoring and assembling regulatory and clinical documents.
However, clinical and regulatory content generation solutions differ significantly from consumer AI tools. Key differentiators include:
Compliance with regulatory and health authorities:
AI-driven solutions for clinical and regulatory content must comply with stringent validation, transparency, data privacy, and quality standards, as well as regulatory requirements from bodies such as the FDA, EMA, and MHRA, alongside international guidelines from ICH.
Data source traceability:
The content generated needs to align with the clinical data. The representation of the data must align with patient-level clinical data sources, like laboratory results, vital signs, and adverse events. These data sources, along with others, comprise the structured content we examined in Part Two. AI-enabled content generation involves the formatting and arrangement of this, as well as creating concise and complete summary narratives. The solution must deliver on quality and compliance with this structured content.
Specific clinical and regulatory use cases:
Many of the vendors we partner with specialize in generative AI for specific document types, including Investigator Brochures, Statistical Analysis Plans, Briefing Documents, Health Authority Responses, Publications, Common Technical Document, Protocols, and Informed Consent Forms. The machine learning component of generative AI leads solution providers to concentrate on specific documents as they develop their solutions with their clients.
The solution market for Clinical and Regulatory AI generation solutions is a rapidly evolving space, full of vendors competing for clients to expand their business and continue to develop their product’s viability.
What to look for in a vendor solution?
Selecting an AI-driven content platform entails more than a feature checklist and a product demonstration. Based on the evaluations we have made, the following criteria are critical for success:
Depth of regulatory content expertise
Ask what their models have been trained on? Is it public data or proprietary datasets? Pharma documents? Vendors should provide evidence of domain-specific knowledge, not generic NLP capability.
Scalability without constant vendor dependence
Can vendor team configure templates, adjust logic/apply rules, or onboard new use cases without involving the vendor every time? If not, you’re buying a bottleneck, not a solution.
Speed and flexibility of enhancements
How quickly can the vendor implement feedback or configuration updates? Prioritize vendors with a track record of responsive iteration and timely delivery, beyond just long-term roadmap commitments.
Ability to scale across use cases without rebuilding
Can the vendor extend existing logic into new document types or does each use case require new builds from scratch?
Pro Tip: True platform vendors offer reusable components and logic layers.
Vendor’s experience and bench strength
What reference work can the vendor share? Do they understand eCTD, regulatory deadlines, and audit-readiness or are they a tech startup still learning space?
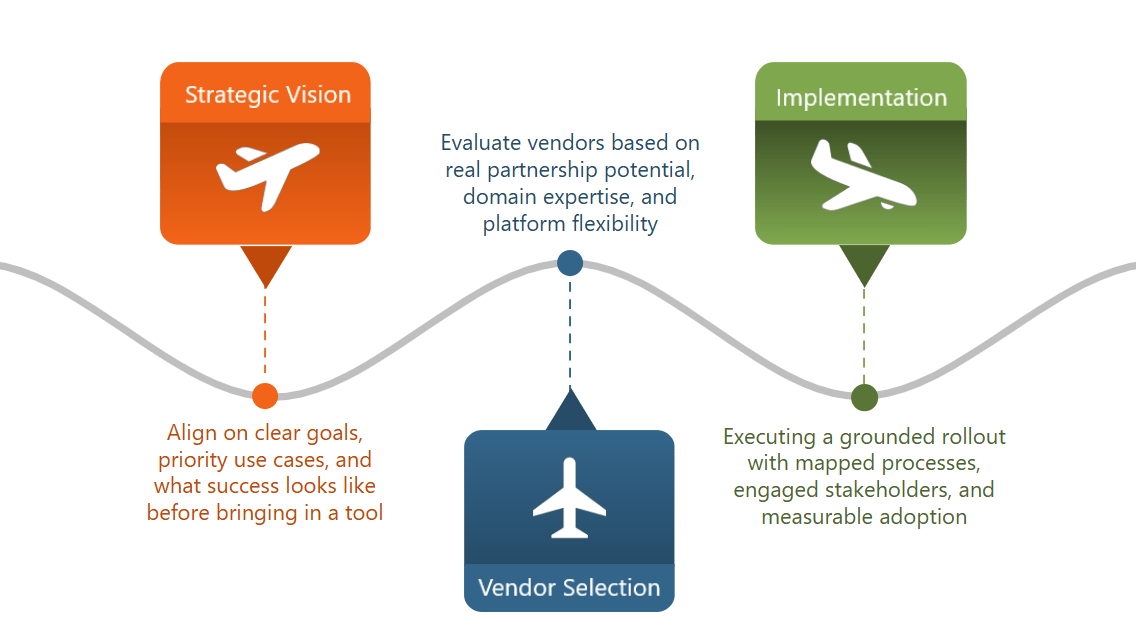
Turning vision into execution:
Building on the foundation of clear user and system functionality requirements, the implementation plan is a critical success factor post-vendor selection. This plan involves a clear assessment of the current state of clinical and regulatory document authoring, surfacing the key stakeholders’ pain points and goals for technology, so the organization can move forward unified around the strategic vision of generative AI and structured content authoring.
Conclusion
AI enabled content generation is no longer a futuristic concept. It is here and rapidly changing how clinical and regulatory teams create documents.
Summary of key takeaways:
The marketplace continues to evolve with emerging capabilities such as real-time content validation, multilingual generation, and integration with advanced analytics on the horizon. The investment priorities for innovation leaders at life sciences companies will drive more investment in Generative AI solutions; “The 2023 Gartner Business Outcomes of Technology by Use Case Survey showed that already 38% of life science respondents were ‘currently utilizing/experimenting with GenAI,’ and a further 44% planned to in the next 12 months. (Gartner, 2024)”
At Astrix, we’ve led implementation efforts across global pharma teams by tailoring blueprints to each organization’s maturity, systems landscape, and change appetite. We partner with our clients to deliver role alignment, success metrics, training, and communication plans. The result is not just adoption, but sustainable, business-aligned transformation.
CITATIONS: Gartner. (2024, January 10). Predicts 2024: Generative AI brings new value to life sciences. Gartner, Inc. https://www.gartner.com/en/documents/5090431
GARTNER is a registered trademark and service mark of Gartner, Inc. and/or its affiliates in the U.S. and internationally and is used herein with permission. All rights reserved.
About Astrix
Astrix is the unrivaled market-leader in creating & delivering innovative strategies, solutions, and people to the life science community. Through world class people, process, and technology, Astrix works with clients to fundamentally improve business & scientific outcomes and the quality of life everywhere. Founded by scientists to solve the unique challenges of the life science community, Astrix offers a growing array of strategic, technical, and staffing services designed to deliver value to clients across their organizations.
Case Study: LabWare Centralized Data Review for a Global Biopharmaceutical Company
Overview A global biopharmaceutical company specializing in discovery, development,... LEARN MOREWhite Paper: Managing Data Integrity in FDA-Regulated labs.
New White Paper LEARN MORELET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and staffing challenges.
CONTACT US



