
FDA Modernization Act 2.0: Accelerate Readiness with an Advanced LIMS Solution
In April 2025, clinical research reached a turning point, with advances reshaping both the science and the regulations that guide it. The FDA unveiled its Roadmap to Reducing Animal Testing in Preclinical Safety Studies, offering a phased strategy for integrating non-animal methods into drug development. Around the same time, the NIH announced the launch of ORIVA—the Office of Research Innovation, Validation, and Application—dedicated to scaling up human-relevant research models. These parallel initiatives highlight a growing consensus: animal models are too often poor predictors of human outcomes, and new technologies can provide safer, faster, and more ethical alternatives.
Drug development has always been a high-risk endeavor, but one of the most striking realities is how often early success fails to translate into human benefit. As the FDA notes in the recently released roadmap, as many as nine out of ten experimental drugs that appear promising in preclinical testing—including animal studies—ultimately fail in human clinical trials, due to safety and/or efficacy issues. And as much as that number is often cited by critics of animal research, the truth is even more complex.
Preclinical success in animal models rarely ensures survival through the rigors of human trials. The reality is that failures continue at every stage of Phase 1 through Phase 3, underscoring just how unpredictable human biology can be. While this challenge isn’t unique to animal-based research, it does highlight the limitations of relying on other species as stand-ins for people.
The implications go beyond science. Each failed drug candidate represents years of research and hundreds of millions of dollars in sunk costs. Animal research also raises enduring ethical questions about the use of large numbers of animals in studies that so often fail to deliver results for people.
The Birth of the FDA Modernization Act 2.0
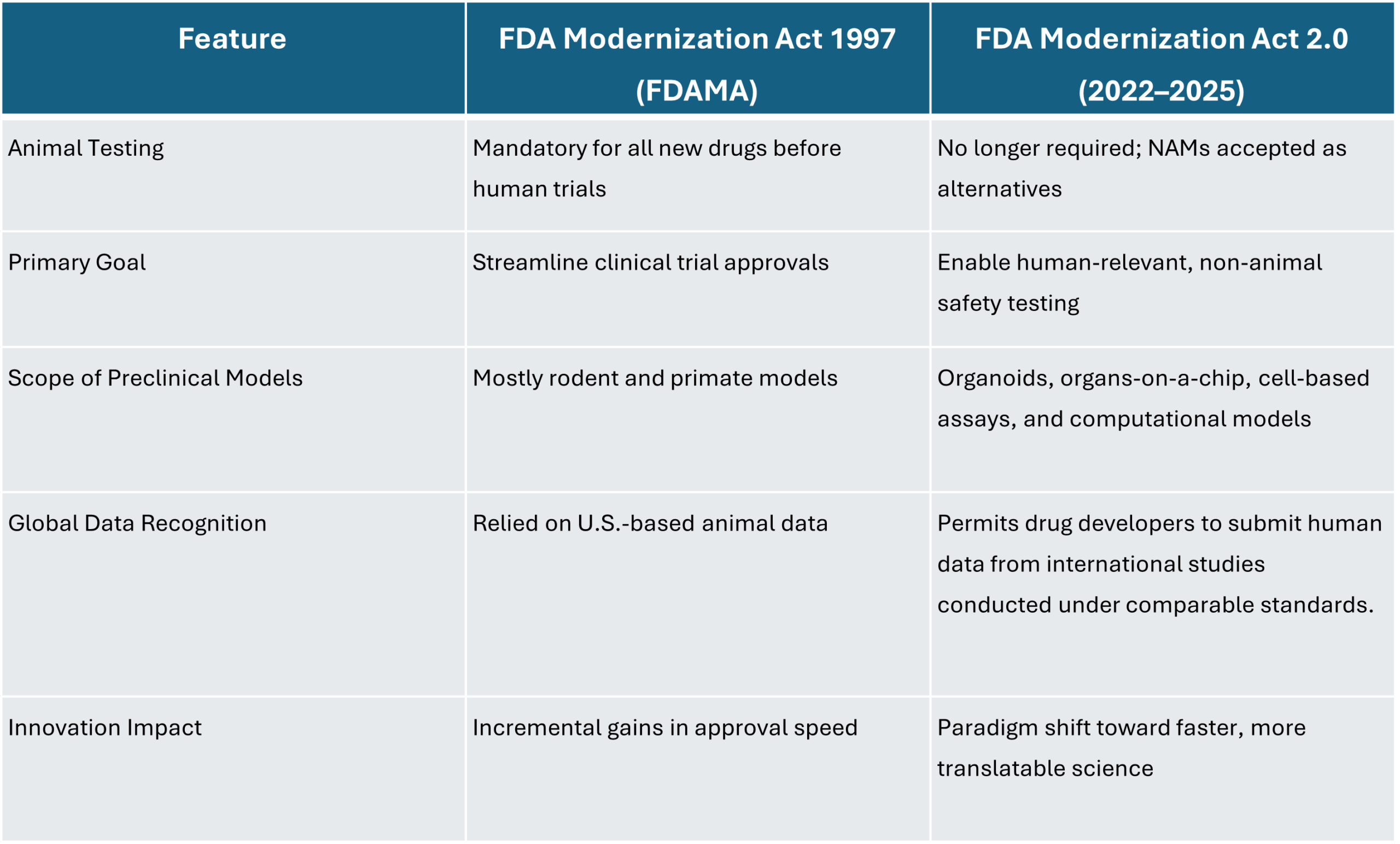
The reliance on animals was not accidental. It was built into the U.S. law beginning with the Federal Food, Drug, and Cosmetic Act of 1938, which made animal testing a legal prerequisite for new drugs. Nearly 60 years later, the FDA Modernization Act of 1997 (FDAMA) streamlined approvals and modernized several aspects of clinical trial oversight, but it did not alter the requirement for animal data. The result was a system where, despite tremendous scientific advances, preclinical drug development remained tethered to models that often did not translate to human outcomes.
This backdrop set the stage for reform. In December 2022, Congress passed and the President signed the FDA Modernization Act 2.0, which for the first time removed the mandate that all new drugs must undergo animal testing before human trials. As Senator Rand Paul noted at the time, animal studies are “expensive and slow” and too often “fail to predict human outcomes.” The new law opened the door for alternative approaches—such as organ-on-a-chip systems, computational simulations, organoids, and cell-based assays—that reflect human biology more directly. By April 2025, the FDA had gone further, making it clear that these methods are not only allowed but expected to play a central role in drug development, particularly for biologics like monoclonal antibodies where animal testing provides limited insight.
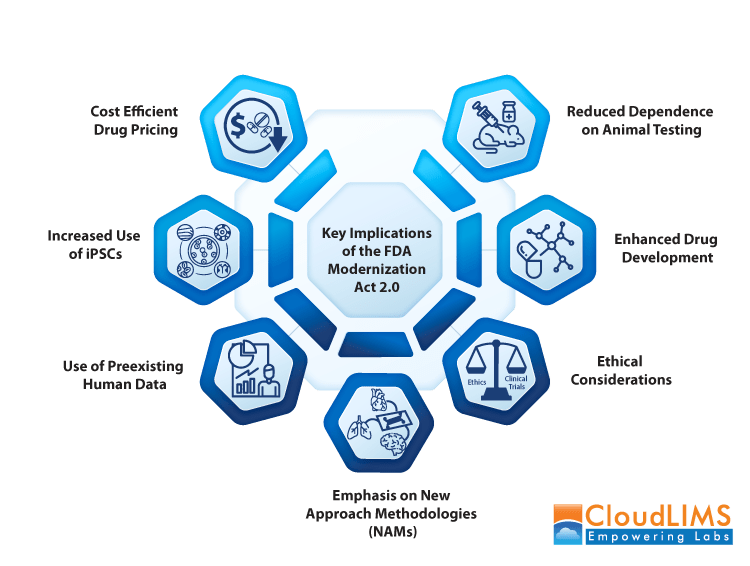
Figure 1: Key impacts of the FDA Modernization Act 2.0 on clinical research laboratories (Image credit: CloudLIMS)
Key Updates in the FDA Modernization Act 2.0
The modernization act introduces what regulators now call New Approach Methodologies (NAMs). These include microphysiological systems (MPS) that replicate organ-level function, induced pluripotent stem cell (iPSC)-derived assays that can mimic human tissues, and AI-based computational models that can analyze massive datasets to predict toxicity and efficacy. As the NIH shifts focus to human-based approaches, Director Jay Bhattacharya remarked that, “For decades, our biomedical research system has relied heavily on animal models. With this initiative, NIH is ushering in a new era of innovation.”
Several core updates stand out:
- Removal of the mandatory animal testing requirement: For the first time since 1938, drug sponsors are no longer legally bound to conduct animal studies before filing for human trials. Instead, they can use scientifically validated alternatives.
- Formal recognition of NAMs: The Act establishes MPS, organoids, iPSC-derived assays, computational modeling, and other innovative technologies as legitimate options for preclinical safety and efficacy testing.
- Broader acceptance of international human data: Data from human studies conducted in countries with comparable regulatory standards can now be used, reducing redundancy and avoiding unnecessary animal testing.
- Encouragement of drug development efficiency: By supporting faster, more predictive testing methods, the Act aims to shorten development timelines, reduce costs, and help life-saving treatments reach patients sooner.
- Ethical modernization: The shift away from compulsory animal studies reflects not only scientific advances but also eliminates ethical concerns about the scale of animal use in drug development.
To better understand how far the law has shifted, consider the following comparison:
For clinical labs, this is more than just a legal update. It is a practical shift that changes how experiments are designed, validated, and reported. A lab running liver toxicity tests, for instance, might now combine an organ-on-a-chip assay with computational metabolic predictions instead of defaulting to rodent studies. A cancer research team might supplement patient-derived organoid data with global human-use data, cutting months from development timelines.
Adapting Laboratory Workflows with an Advanced LIMS Solution
While the modernization act brings exciting opportunities, it also introduces complexity. Labs are no longer working with a single data stream from animal studies but juggling multiple NAMs, each generating its own format of results. Without a system to capture, integrate, and standardize this information, labs risk falling out of compliance or missing key insights.
With this, the role of an advanced Laboratory Information Management System (LIMS) solution becomes evident. By automating workflows, a LIMS allows labs to handle the influx of diverse data types seamlessly. It ensures that every organoid culture plate, chip-based dataset, and computational model output is traceable and linked to a secure audit trail. Integration with instruments and software eliminates manual data entry errors, while configurable workflows allow labs to adapt quickly as new NAM technologies emerge.
A cloud-based advanced LIMS solution offers even more flexibility. It gives remote teams centralized access to trial data, simplifies collaboration across institutions, and makes regulatory submissions more straightforward. With features like multi-factor authentication, role-based permissions, electronic signatures, and customizable reports, a modern LIMS provides both the security and adaptability that labs need as they transition into the FDA 2.0 era.
Imagine a CRO supporting multiple sponsors. One of the CRO’s projects requires tumor organoid assays, another focuses on AI-driven toxicity predictions, and a third uses patient-derived iPSC models. A traditional data management approach would collapse under this diversity. With a configurable and advanced clinical research LIMS, the lab can run all three projects in parallel, manage heterogeneous data generated from each project, maintain compliance across the board, and deliver results faster than ever before.
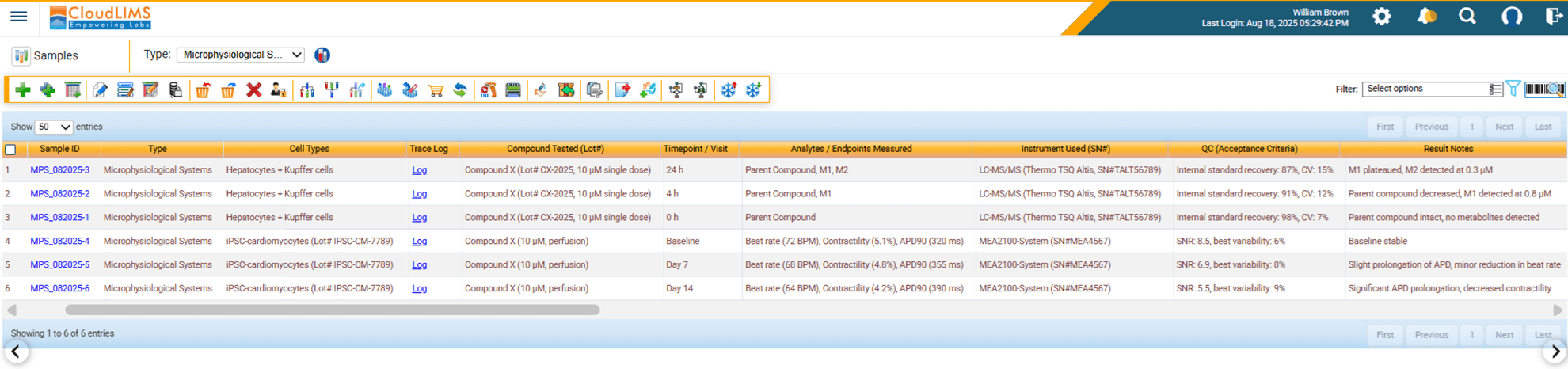
Figure 2: Easily manage large volumes of clinical samples and metadata with a LIMS
Final Thoughts
By moving away from animal testing mandates and embracing NAMs, the FDA Modernization Act 2.0 paves the way for research that is more ethical, more efficient, and more relevant to human biology.
The task of translating this shift into day-to-day research will fall heavily on clinical labs. To succeed, they must not only adopt the new technologies but also manage them with precision. A modern LIMS is the backbone that makes this possible, offering the flexibility, traceability, and compliance support needed to thrive in this new era.
As compulsory animal studies fade into the past, the labs that embrace human-based NAMs—and the digital systems to support them—will lead the way into a future where innovation is faster, costs are lower, and treatments reach patients sooner.
About CloudLIMS
CloudLIMS.com offers a secure, cloud-based SaaS LIMS with zero upfront cost for a wide range of industries. It helps biorepositories, clinical research and diagnostic laboratories, and analytical testing laboratories—such as those in environmental testing, materials and mining, food and beverage, and cannabis testing—manage data, automate workflows, and meet regulatory compliance and standards such as US FDA, EMEA, CAP, EU GDPR, ICH-GCP, HIPAA, ISO/IEC 17025, ISO 20387, NELAC, 21 CFR Part 11, and local regulations. CloudLIMS also provides a range of complimentary services, including technical support, product training, instrument integration, reporting templates, product upgrades, legacy data migration, and automatic data backups. Its mission is to digitally transform and empower laboratories worldwide to improve the quality of life. CloudLIMS.com is a SOC 2 compliant and ISO 9001:2015 certified laboratory informatics company.
About the Author
Shonali Paul, COO, CloudLIMS

Shonali Paul has a rich experience of working in diverse industries including IT, heavy engineering, and retail. In a career spanning over 24 years, she has built a long and impressive track record of success in high technology software sales, marketing, and professional services, developing operational strategies, and directing new business initiatives from conception through execution.
She has helped build the offshore development center in India and is the key driver of the development, operations, and product teams. She has been the key person responsible for two corporate acquisitions. She served as the Member Relations Committee chair at the International Society for Biological and Environmental Repositories (ISBER) for two terms.
She has been extensively published in international publications. She is an invited speaker at conferences across the globe and has delivered insightful presentations at numerous international events.
Shonali Paul holds a Bachelor of Engineering degree from SGSITS, Indore, and an MBA from Xavier Institute of Management, Bhubaneswar, India.
About Astrix
Astrix partners with many of the industry leaders in the informatics space to offer state-of-the-art solutions for all of your laboratory informatics needs. Our technology-agnostic approach delivers tailored solutions and expert talent to address even the most complex challenges. With deep expertise in system validation, data integrity, and risk management, we streamline implementation, ensure compliance, and identify system gaps to minimize long-term risks and costs. Whether you need strategic planning, technology integration, or staffing support, Astrix is your trusted partner for driving business success. Reach out today at www.astrixinc.com to learn how we can accelerate your growth and optimize operations with our specialized life science solutions.
Case Study: LabWare Centralized Data Review for a Global Biopharmaceutical Company
Overview A global biopharmaceutical company specializing in discovery, development,... LEARN MOREWhite Paper: Managing Data Integrity in FDA-Regulated labs.
New White Paper LEARN MORELET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and strategic talent services.
CONTACT US



